HIV infection rates have grown in Australia by 50 per cent over the last 10 years with health researchers believing up to 10,000 people in Australia have undiagnosed HIV leading one peak HIV body saying undiagnosed HIV infection and inadvertent transmission would drop dramatically if rapid HIV test kits were approved for sale there. According to an annual HIV surveillance report HIV infection rates in Australia grew by 8 per cent in 2011 with 1,137 new HIV diagnoses in 2011, compared with 719 in 1999. At the same time sexually transmitted diss eases such as gonorrhea and chlamydia are also being increasingly detected. Professor John de Wit, director of the National Centre in HIV Social Research, said a further 30 … Continue reading
John Le Fevre

Australian consumers are unlikely to see oral HIV rapid tests on sale in the near future with the country’s regulating body, the Therapeutic Goods Administration (TGA), saying by law it is not allowed to evaluate applications to supply HIV home test kits. At the same time Levinia Crooks, chief executive of the Australasian Society for HIV Management, says that while the OraQuick rapid HIV instant test kit by OraSure Technologies is relatively easy to self-administer, it is not a highly sensitive test. Earlier this week the US-FDA approved the OraQuick rapid HIV instant test kit for over-the-counter (OTC) sales in the USA, despite researchers finding that in home HIV testing use the oral HIV rapid tests returned a Specificity of … Continue reading
The US-FDA (Food and Drug Administration) has approved the OraQuick rapid HIV instant test kit by OraSure Technologies as the first rapid HIV home test kit available for OTC (over the counter) purchase in the United States. The approval will enable Americans to purchase the OraQuick rapid HIV instant test and ascertain their HIV infection status in the privacy of their homes, with the OraQuick rapid HIV home test providing results in between 20 and 40 minutes from a saliva mouth swab. AIDS researcher and director of the National Institute of Allergy and Infectious Diseases, Dr. Anthony S. Fauci, said the freer availability of rapid HIV instant test kits for home HIV testing could help bring the 30-year-old epidemic under … Continue reading

British pharmaceutical manufacturer GlaxoSmithKline has agreed to plead guilty to criminal charges in the US and pay $US3 billion in fines for promoting its best-selling antidepressants for unapproved uses and failing to report safety data about a top diabetes drug. According to US federal prosecutors the deal with GlaxoSmithKline, the world’s fourth-largest pharmaceutical company measured by 2009 prescription drug sales after Pfizer, Novartis and Sanofi, also includes penalties for it improperly marketing half-dozen other drugs and comes on top of a $1.6 billion fine levied in May on Abbott Laboratories over the marketing of the antipsychotic drug Depakote, while an agreement with Johnson & Johnson over its off-label promotion of another antipsychotic drug, Risperdal, could see the pharmaceutical giant fined … Continue reading
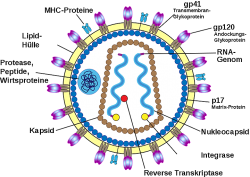
An analysis of the modestly successful Phase III RV144 clinical HIV vaccine trial in Thailand between 2003 and 2006 involving more than 16,000 people has produced new information that may assist in the development of a broadly protective HIV vaccine. The RV144 HIV vaccine trial led by Supachai Rerks-Ngarm, MD., of the Thai Ministry of Public Health’s Department of Disease Control and supported by the U.S. Army in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), tested the safety and effectiveness of a prime-boost regimen of two vaccines, a modified canarypox vaccine, ALVAC-HIV (the primer dose), and a glycoprotein 120 vaccine, AIDSVAX B/E (the booster dose), on volunteers aged between 18 and 30 years of age in … Continue reading